Zika Virus (ZIKV) is an arbovirus belonging to the Flaviviridaetransmitted primarily by mosquitoes (including Aedes Agypti and Aedes albopictus). Although first identified in a rhesus monkey from the forests in Uganda in the year 1947 -with an estimated first emergence probably in 1920- the first human case was only reported in 1952 in Nigeria. ZIKV has been shown to be distributed Northern Africa as well as in Southeast Asia and the Pacific; however only a few human cases in Africa and Asia were identified until 2007, when a ZIKV outbreak was reported in Yap/Micronesia followed by an outbreak in French Polynesia and New Caledonia 2019 and currently in Central and South America as well as the Caribbean.
Most epidemiological studies that are based on the seroprevalence of neutralizing antibodies, suggest that up to 73% of the population (6.1-73%) have been exposed to ZIKV.
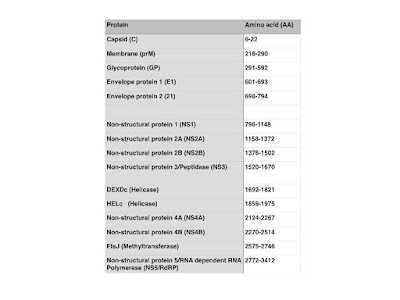
Being a Flavivirus, ZIKV -like the related West Nile Virus (WNV), Japanese Encephalitis Virus (JEV), Chikungunya (CHIKV) and Dengue Virus (DENGV)- has a single strand, positive sense RNA genome, encoding for a polyprotein that ultimately is processed into three structural proteins, the Capsid (C), precursor of Membrane protein (prM), and Envelope (E) protein as well as seven non-structural proteins (NS1-5).
Similar to CHIKV, the infection with ZIKV is a relative mild disease, characterised symptoms ranging from mild fever, headaches, conjunctivitis, maculopapular rashes, vertigo, or myalgia with low mortality and often is asymptomatic.
Since the clinical presentation is similar to infections with other arboviruses, in particular CHIKV and DENGV, diagnostics is difficult and mostly done by using RT-PCR of samples that are CHIKV and DENGV negative. Although serological tests (ELISA, Immunofluorescence) have been used in the past, they are less reliable due to cross-reactivity with other flaviviruses such as DENGV or Yellow Fever Virus. Currently no commercial kit is available to test for ZIKV in patients.
Being transmitted by infected mosquitoes, the initial target cells are in the skin compartment, both skin fibroblasts, epidermal keratinocytes and dendritic cells are highly permissible for ZIKV which enters the host cell a number cellular receptors, in particular DC-SIGN, AXL, Tyro-3, and (albeit to a lesser extent) TIM-1. Primary skin fibroblasts infected with ZIKV support viral replication whereas in infected primary epidermal keratinocytes, similar to keratinocytes infected with DENV, exhibit large cytoplasmic vacuolation and pyknotic nuclei can be observed, suggesting that ZIKV induces apoptosis in these cells despite supporting viral replication.
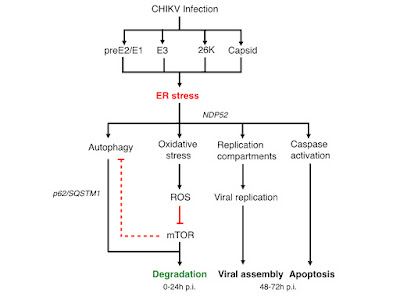
Similar to JEV and CHIKV infected cell, ZIKV induces the formation of autophagosome-like structure that form the scaffold for the assembly of viral replication centres. If the expression of the viral preE2/E1 and Capsid proteins also induces ER stress (similar to CHIKV) and if the ER stress induced by ZIKV contributes to the induction of autophagy and/or caspase dependent apoptosis has not been demonstrated yet, but seems to be very likely.
Likewise it has not been demonstrated if the formation of autophagosomes by ZIKV in primary skin fibroblasts is -as in the case of CHIKV- dependent on LC3-C and NDP52. Given that only the Asian lineage, in contrast to the African lineage, of ZIKV has been able to cause prolonged epidemics in the human populations (in 2007 and currently in the Americas) it has been speculated that the viral NS1 gene adaptions increases viral fitness in humans. Therefore, it might be necessary to investigate the ability of different isolates from different lineages to induce the formation of replication centres in primary human cells. If the decrease in autophagic flux or viral induced ER stress in infected primary dermal and skin cells or in keratinocytes induces apoptosis has not been demonstrated.
 |
| Figure: CHIKV and the ER stress response |
 |
| Figure: JEV and the ER stress response |
 |
| Figure: JEV and the induction of autophagosome formation via non-structural and structural proteins located at the ER |
Likewise it has not been demonstrated if the formation of autophagosomes by ZIKV in primary skin fibroblasts is -as in the case of CHIKV- dependent on LC3-C and NDP52. Given that only the Asian lineage, in contrast to the African lineage, of ZIKV has been able to cause prolonged epidemics in the human populations (in 2007 and currently in the Americas) it has been speculated that the viral NS1 gene adaptions increases viral fitness in humans. Therefore, it might be necessary to investigate the ability of different isolates from different lineages to induce the formation of replication centres in primary human cells. If the decrease in autophagic flux or viral induced ER stress in infected primary dermal and skin cells or in keratinocytes induces apoptosis has not been demonstrated.
Antiviral response induced by ZIKV : role of the antiviral interferon response
In primary skin cells, following viral entry and release of the viral genome, the viral RNA induces the transcription of Interferon stimulated genes (ISG) such as OAS2, MX1, and ISG15, as well as the transcription of RIG-1, MDA-5, and Toll-like receptor (TLR-3) in a Interferon-β (IFN-β).As a result, viral replication is inhibited.
Since the infection of primary human dermal fibroblasts and primary human foreskin fibroblast cells (HFF) with ZIKV also induces the formation of LC3-II positive membrane vesicles , it might be possible that TLR-3 mediated NF-κB signaling induces autophagy in a DRAM-1 dependent manner.
If these vesicles however represent viral replication centres or are involved in antiviral signaling by degrading viral components (or are even involved in the presentation of viral antigens via the MHC- Class I and II complexes) is not clear.
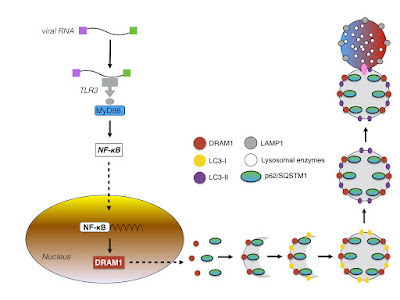 |
| Figure: TLR-3 and autophagy; formation of autophagosomes via DRAM-1 |
The intracelebral inoculation of mice with ZIKV results in productive viral replication in both neuronal and astroglial cells, with autophagosome-like structures present that resemble viral replication centres. A subset of infected cells however has been shown to undergo necrosis, suggesting that the infection of neuronal cells with ZIKV -similar to human medullablastoma TE-671 cells, astrocytes and neuronal cells infected with JEV- induces both the formation of autophagosome-like structures as well as cell death. If however ZIKV induced cell death is dependent on the induction of caspase-3 and -9 in addition to ROS dependent activation of apoptosis signaling kinase (ASK)-1 and p38 MAPK signaling pathways has not been demonstrated. Despite the close proximity of ZIKV replication centres, an involvement of the ER stress response in the formation of ZIKV RC has not been demonstrated as well.
ZIKV and the nucleolus
As discussed in a previous post, a number of viral proteins localises to the nucleolus, thus (potentially) inducing the formation of autophagosomes and/or inducing via activation of p53. In the case of the small isoform of West Nile Virus (WNV) Capsid protein for instance, p53 dependent apoptosis is induced by sequestration of Hdm2 to the nucleolus whilst the large isoform inhibits apoptosis and activates mTOR-dependent p70S6K, thus inducing translation of viral genes. If either isoform, affects also the formation of LC3-II positive vesicles has not been demonstrated; it should be noted however that the NS4A and NS4B proteins from all WNV isolates (except NY99) induce autophagy as a result of ER stress. Since the NS4B protein derived from the WNV Kunjin subtype also localises to the nucleolus, an involvement of the nucleolus in the formation of WNV RC cannot be ruled out.
It might therefore possible that the nucleolar localisation of ZIKV protein(s) might induce a cell cycle delay, promote apoptosis or induce autophagy.
 |
| Table3: Flavivirus proteins that localise to the nucleolus |
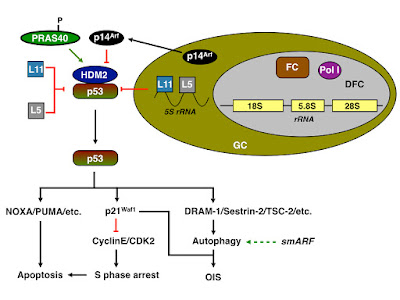 |
| Figure: ZIKV might promote autophagy or induce apoptosis by sequestering p53 |
ZIKV, Microcephaly, and Guillan-Barre Syndrome
In the case of viral induced microcephaly, in uteroinfection with Human Cytomegalovirus (HCMV) has been associated with cases of microcephaly in newborn infants, but if ZIKV is a causative agent of microcephaly as well or if other factors -such as co-infection of pregnant women with other neurotrophic viruses such as DENGV or CHIKV, and/or parasites such as malaria as well as environmental factors despite the isolation of viral RNA from the placenta and amniotic fluid also play a role is not clear.
During the current outbreak of ZIKV in the Americas as well as during the outbreak of ZIKV in French Polynesia, an increase in patients exhibiting Guillan-Barre Syndrome (GBS), a rare neurological disorder caused by an autoimmune response, has been reported. Although so far no direct link between ZIKV and GBS has been reported, it might be possible that the decrease in autophagic flux in ZIKV infected neuronal cells induces apoptosis or if the observed increase is related to an autoimmune response as a result of the immune response (as in cases of rheumatoid arthritis in CHIKV positive patients) remains to be seen.
In summary, based on results published on other members of the Flaviviridae, ZIKV might exhibit similar features regarding the interaction with host cell, including the formation of replication centres by inducing the ER stress response, the localisation of viral proteins to the nucleolus and -probably most importantly-the induction of antiviral Interferon signaling via the induction of TLR-3 mediated NF-κB signaling (that might also induce the formation of autophagosomes, supporting viral replication and/or degrading viral proteins and RNA) and the induction of Interferon stimulated genes (ISG), thus inhibiting viral replication.
Further reading
Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, & Herida M (2019). Current Zika virus epidemiology and recent epidemics. Medecine et maladies infectieuses, 44 (7), 302-7 PMID: 25001879
Jan C, Languillat G, Renaudet J, & Robin Y (1978). [A serological survey of arboviruses in Gabon]. Bulletin de la Societe de pathologie exotique et de ses filiales, 71 (2), 140-6 PMID: 743766
Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, & Leroy EM (2019). Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS neglected tropical diseases, 8 (2) PMID: 24516683
Kuno G, & Chang GJ (2007). Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Archives of virology, 152 (4), 687-96 PMID: 17195954
Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Desprès P, Amara A, Yssel H, & Missé D (2019). Biology of Zika Virus Infection in Human Skin Cells. Journal of virology, 89 (17), 8880-96 PMID: 26085147
Choi SH, Gonen A, Diehl CJ, Kim J, Almazan F, Witztum JL, & Miller YI (2019). SYK regulates macrophage MHC-II expression via activation of autophagy in response to oxidized LDL. Autophagy, 11 (5), 785-95 PMID: 25946330
Gannage M, da Silva RB, & Münz C (2019). Antigen processing for MHC presentation via macroautophagy. Methods in molecular biology (Clifton, N.J.), 960, 473-88 PMID: 23329508
Chemali M, Radtke K, Desjardins M, & English L (2011). Alternative pathways for MHC class I presentation: a new function for autophagy. Cellular and molecular life sciences : CMLS, 68 (9), 1533-41 PMID: 21390546
Gannage M, & Münz C (2010). MHC presentation via autophagy and how viruses escape from it. Seminars in immunopathology, 32 (4), 373-81 PMID: 20857294
Yang TC, Shiu SL, Chuang PH, Lin YJ, Wan L, Lan YC, & Lin CW (2009). Japanese encephalitis virus NS2B-NS3 protease induces caspase 3 activation and mitochondria-mediated apoptosis in human medulloblastoma cells. Virus research, 143 (1), 77-85 PMID: 19463724
Bell TM, Field EJ, & Narang HK (1971). Zika virus infection of the central nervous system of mice. Archiv fur die gesamte Virusforschung, 35 (2), 183-93 PMID: 5002906 Tetro JA (2019). Zika and microcephaly: Causation, correlation, or coincidence? Microbes and infection / Institut Pasteur PMID: 26774330
Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, & Tesh RB (2011). Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerging infectious diseases, 17 (5), 880-2 PMID: 21529401
Salvetti A, & Greco A (2019). Viruses and the nucleolus: the fatal attraction. Biochimica et biophysica acta, 1842 (6), 840-7 PMID: 24378568
Xu Z, Anderson R, & Hobman TC (2011). The capsid-binding nucleolar helicase DDX56 is important for infectivity of West Nile virus. Journal of virology, 85 (11), 5571-80 PMID: 21411523
Katoh H, Mori Y, Kambara H, Abe T, Fukuhara T, Morita E, Moriishi K, Kamitani W, & Matsuura Y (2011). Heterogeneous nuclear ribonucleoprotein A2 participates in the replication of Japanese encephalitis virus through an interaction with viral proteins and RNA. Journal of virology, 85 (21), 10976-88 PMID: 21865391
Buckley A, & Gould EA (1988). Detection of virus-specific antigen in the nuclei or nucleoli of cells infected with Zika or Langat virus. The Journal of general virology, 69 ( Pt 8), 1913-20 PMID: 2841406
Westaway EG, Khromykh AA, Kenney MT, Mackenzie JM, & Jones MK (1997). Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology, 234 (1), 31-41 PMID: 9234944
Buckley A, Gaidamovich S, Turchinskaya A, & Gould EA (1992). Monoclonal antibodies identify the NS5 yellow fever virus non-structural protein in the nuclei of infected cells. The Journal of general virology, 73 ( Pt 5), 1125-30 PMID: 1534119
Yang MR, Lee SR, Oh W, Lee EW, Yeh JY, Nah JJ, Joo YS, Shin J, Lee HW, Pyo S, & Song J (2008). West Nile virus capsid protein induces p53-mediated apoptosis via the sequestration of HDM2 to the nucleolus. Cellular microbiology, 10 (1), 165-76 PMID: 17697133
Shives KD, Beatman EL, Chamanian M, O'Brien C, Hobson-Peters J, & Beckham JD (2019). West nile virus-induced activation of mammalian target of rapamycin complex 1 supports viral growth and viral protein expression. Journal of virology, 88 (16), 9458-71 PMID: 24920798
Urbanowski MD, & Hobman TC (2019). The West Nile virus capsid protein blocks apoptosis through a phosphatidylinositol 3-kinase-dependent mechanism. Journal of virology, 87 (2), 872-81 PMID: 23115297
Blázquez AB, Martín-Acebes MA, & Saiz JC (2019). Amino acid substitutions in the non-structural proteins 4A or 4B modulate the induction of autophagy in West Nile virus infected cells independently of the activation of the unfolded protein response. Frontiers in microbiology, 5 PMID: 25642225
Martín-Acebes MA, & Saiz JC (2011). A West Nile virus mutant with increased resistance to acid-induced inactivation. The Journal of general virology, 92 (Pt 4), 831-40 PMID: 21228127 Martín-Acebes MA, Blázquez AB, & Saiz JC (2019). Reconciling West Nile virus with the autophagic pathway. Autophagy, 11 (5), 861-4 PMID: 25946067
Naing ZW, Scott GM, Shand A, Hamilton ST, van Zuylen WJ, Basha J, Hall B, Craig ME, & Rawlinson WD (2019). Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. The Australian & New Zealand journal of obstetrics & gynaecology, 56 (1), 9-18 PMID: 26391432